Whitepaper
Companion Diagnostic Assays: Global Market and Emerging Opportunities
Companion Diagnostic Assays
Overview
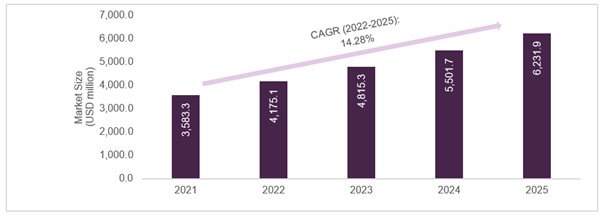
As a central driving force for precision medicine worldwide, the Personalized Medicine Coalition (PMC) noted that more than 220 FDA-approved therapies with a biomarker mentioned in the label as ‘essential’ or ‘recommended for prescribing’ were available in the US market between 2015 and 2021. Further fuelling the movement of value-based care, a drug-diagnostic co-development model has been significantly prevalent, leading to a consistently growing usage of companion diagnostics assays – to target patients expected to benefit most from targeted therapy. As of 2022, 40+ commercialized CDx assays were listed exhaustively in the FDA’s List of Cleared or Approved Companion Diagnostic Devices.
Although there has been a significant improvement in the dissemination of patient care due to targeted management regimes, most healthcare providers still utilize tissue biopsies for CDx tests. This may often lead to scheduling limitations, insufficient tissue samples, patient aversion, repeat biopsies, and long turnaround times, often hindering timely diagnosis. However, liquid biopsies, using plasma circulating tumor DNA, have the potential to overcome restrictions imposed by tissue-based testing. The minimally invasive nature of liquid biopsies provides greater convenience and patient acceptance, spurring multiple diagnostic companies to tap into the emerging market.
Publication
Download the publication
Gain a competitive edge and stay informed about the latest industry trends by downloading our comprehensive whitepaper.