Launching any product in a new market can be challenging, and when that product must conform with complex regulations designed to protect human health and the environment, having a good understanding of those regulation processes is a must. Pesticides are one such highly regulated and scrutinized type of product. There are challenges to overcome when developing and launching a pesticide into a new market or when you are extending existing product formulations and lines in a current market. Each regulated market in a specific country can have differing information requirements, have different risk sensitivities, and adapt and change regulations in different timeframes. Partnering with experts in product safety regulations can help you maintain compliance and smooth out the regulatory assessment and application process. Here at Evalueserve we are helping clients to throw light on to regulatory frameworks and helping to deliver safe pesticide products to market. We present our review of the Canadian pesticide regulatory pathway, its requirements, and various scenarios that may impact upon your product when registering it for market launch.
Canada Pesticide Registration Regulation Process
In Canada, pesticides are regulated by Health Canada through a system shared by federal, provincial, and municipal governments. Considering the major impact that pesticides can have on human health and the environment a thorough scientific and risk management framework is used to protect both humans and the environment.
Tiers of organizational control in Canada
- Federal Government – Evaluation and registration of pesticides as per the Pest Control Products Act (PCPA) and Regulations.
- Provincial and Territorial Governments – regulate and support the sale, use, storage, transportation, and disposal of registered pesticides. Also support with the education and training of the concerned population, followed by certification and licensing of applicators, vendors, and growers.
- Municipal / Local governments – Regulate the use of pesticides by setting bylaws for that municipal region (and often including private and residential). E.g., “cosmetic use” of certain pesticides is banned only in those municipal regions (cosmetic use – use of pesticides in lawn, turf, and garden to increase the attractiveness).
The Health Canada Pest Management Regulatory Agency (PMRA) created in 1995 is responsible for pesticide regulation in Canada. As a branch of Health Canada, PMRA consolidates the resources and responsibilities for pest management regulation. Pesticides are primarily regulated under the Pest Control Products Act (PCPA) based on scientific risk assessment and risk management.
Before a pesticide can be registered for sale in Canada, pesticide manufacturer applicants are required to provide the PMRA with extensive scientific data to show that their product does not pose unacceptable risks to human health and the environment, and that the product has value.
Regulatory process overview
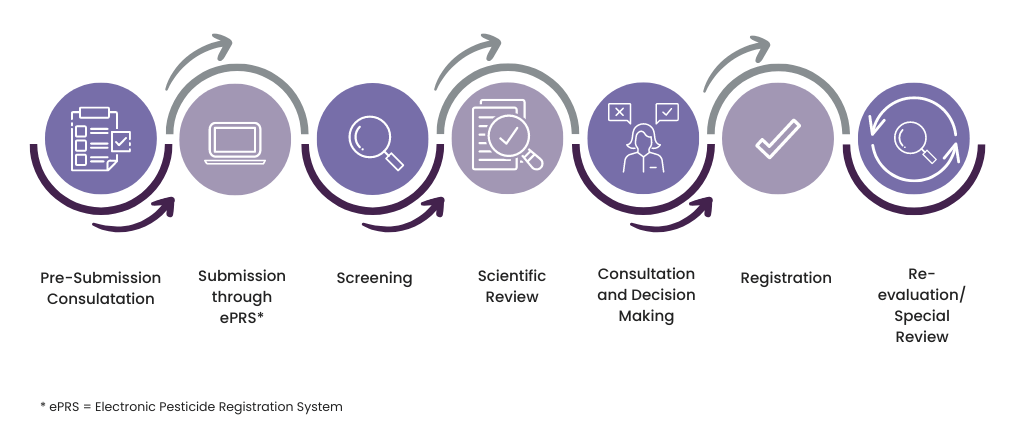
Additionally, there are other acts and regulations which might not be directly related to pesticides but still impact the use of pesticides in Canada.
Pesticide registration in Canada
Pesticide registration in Canada is a multi-step process involving submission, scientific review, and approval. PMRA’s program pre-submission consultation aids new registrants with all the necessary information regarding the registration and amendment process for pesticides in Canada. The pre-submission process may also be utilized as a mechanism for obtaining guidance on a study protocol design. Pre-submission consultations are recommended for:
- Joint Review requests
- Microbial applications
The Electronic Pesticide Regulatory System is a system through which new registrations, amendments, or renewal applications can be submitted to the PMRA. Applicants and Registrants must create an e-Index in PRZ format using the e-Index Builder of the documents they are submitting, and if electronic versions are available, they will be required to package that set of documents into the e-Index.
PMRA’s science-based risk assessment involves a team of scientists evaluating the health and environmental risks of the pesticide. Risk assessment also considers the value of the pesticide and product chemistry to determine if they are acceptable. PMRA’s science-based risk assessment includes the following:
- an examination of all sources and routes (oral, dermal, or inhalation) of potential exposure to a given pesticide
- an estimation of the number of pesticides that people, including children, may be exposed to.
- a laboratory evaluation of product chemistry data.
- a human health risk assessment with a focus on vulnerable populations, including pregnant women, infants, children, women, and seniors.
- an environmental risk assessment that considers the fate of the pesticide including movement, persistence, transformation, toxicity, and risks to terrestrial and aquatic life.
- a value assessment that considers the contribution of the product to pest management, other benefits, and socio-economic impact.
Consultation and Decision Making
Upon completion of the science-based evaluation, the findings undergo a peer review approval. For new active ingredients or major new uses, these are presented to PMRA’s Science Operations Committee for discussion and then to Science Management Committee for decision.
At the end of the review stage, bilingual revised labels are sent to applicants by e-mail to provide an opportunity to comment and clarify issues arising from the revisions. A bilingual consultation document is published on the Health Canada website for all major decisions which outlines major findings and the proposed decision. All comments received from the consultation period are considered before a final decision is made. The final decisions are posted on the Health Canada website.
The Canadian Food Inspection Agency (CFIA) is responsible for monitoring MRL compliance in foods in the Canadian marketplace. Health Canada sets science based MRLs to ensure the food Canadians eat is safe. As of December 2020, Canada had approximately 24,000 pesticide MRLs set.
Re-evaluation and Special Review
As part of the post-market program, registered pesticides are re-evaluated on a cyclical basis once every 15 years, using modern assessment techniques and current scientific information. Also, a pesticide can be re-evaluated if there is any update in the procedure of risk assessment or changes in information requirements.
A special review may also be initiated at any time if there are reasonable grounds to believe that the health or environmental risks, or the value of a pesticide are no longer acceptable. This is targeted to address the specific aspects of concern related to the pest control product that prompted the review. Also, if an OECD member country prohibits all uses of an active ingredient for health or environmental reasons a special review will be initiated.
As part of its commitment to improving transparency, PMRA is publishing interim updates to its five-year Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2020–2025.
New active ingredients and products registered in 2020–2021
In 2020–2021, 10 new active ingredients (the substance with the pesticide effect) were registered for use in Canada, resulting in the registration of 13 new related end-use products (different formulations of products containing the active ingredient).
Joint Review
A formal process where the review of a pesticide dossier is shared by two or more countries. In the last two decades, Canada has progressed from developing pilot pesticide joint review approaches with the United States, to conducting joint reviews as a primary course of business for pre-market reviews. Out of the 10 active ingredients registered in 2020-21, three were joint reviews.
Pesticide Public Registry
Pesticide Public Registry was set up to increase transparency in the pesticide registration system. The Public Registry is a collection of non-confidential information on pesticides and the pesticide regulatory system. The Pesticide Product Information Database allows the public to view information on products, active ingredients, and programs related to pesticides and other pest control products that are regulated by Health Canada.
The reports in the database are grouped by:
- Applications (e.g., Application details for: 2022-1354)
- Products (e.g., Product details for: ACURON HERBICIDE)
- Active ingredients (e.g., Ingredient details for: S-METOLACHLOR AND R-ENANTIOMER)
- Incident Reports (e.g., Incident report details)
- Label Search (e.g., Label details for: ACURON FLEXI HERBICIDE)
- Maximum Residue Limit (MRL) (Maximum residue limits – DAMINOZIDE)
Incident reporting
PMRA uses incident reports to identify and characterize potential risks to humans, domestic animals, and the environment from the use of pesticides, which were not evident during the initial registration of a pesticide. Pesticide registrants are required by law to report all incidents related to their products to Health Canada. In the 2020–2021 fiscal year, 1437 pesticide incident reports and 52 scientific studies were submitted to PMRA.
Health Canada’s compliance and enforcement
Health Canada’s Pesticide Compliance Program is responsible for promoting, monitoring, and enforcing compliance with the Pest Control Products Act and Regulations. The Pesticide Compliance Program has oversight on all parties regulated by the Pest Control Products Act, including pesticide registrants, manufacturers, importers, retailers, and users, and conducts a variety of compliance verification and compliance promotion activities within all sectors. Compliance verification includes carrying out inspections and collection of samples to assess compliance.
In 2020-21 a total of 1336 enforcement actions addressing single or multiple violations were issued to non-compliant parties. The most common violation types noted were importation of unregistered products (29%), sale of unregistered products (20%), and advertising of pest control products in a way that is contrary to the Pest Control Products Act (16%).
Provincial Laws Prohibiting Non-Essential Pesticide Use
The cosmetic use of pesticides is banned in various provinces for uses ranging from broad to narrow in scope. Bans that are considered broad in scope prohibit the cosmetic use of pesticides on all landscaping elements (e.g., Ontario, Quebec, Nova Scotia), while narrowly scoped bans tend to prohibit the cosmetic use of pesticides on lawns only (e.g., Manitoba, New Brunswick, PEI, Newfoundland, and Labrador).
Recent Updates from Canada PMRA
In 2020–2021, PMRA continued work on other regulatory modernization initiatives on the roadmap, including those related to the post-market review process, labeling, data protection, and the authorization of pesticides not requiring registration. PMRA is currently advancing a Transformation Agenda under the Discussion Document DIS2022-01, Further Strengthening Protection of Health and the Environment: Targeted Review of the Pest Control Products Act. This includes several initiatives to change programs intended to:
- Strengthen human health and environmental protection through modernized business processes for the review of pesticides.
- Improve transparency and public access to information and data across the regulatory pesticide processes.
- Increase the use of comprehensive, real-world data on water monitoring, crop production, and pesticide use, as well as independent scientific advice.
To support the implementation of these transformation initiatives, and in alignment with the Government’s commitments, this targeted review of the related provisions of the Pest Control Products Act is being undertaken.
How Evalueserve can support
The Chemical Safety & Regulatory Affairs (CSRA) at Evalueserve is a team with over 50 professionals including, toxicology consultants, toxicology analysts, chemistry experts, regulatory specialists, and business solution architects who can support and provide expert solutions at every stage from the product development phase to launch of the product into the market. Our team is equipped with skills and knowledge to assist you by performing hazard identification and data summarization, undertaking human and environmental risk assessments, and providing you with regulatory intelligence support. If you want to learn more how we can help you with developing and launching pesticide products, then please contact us.
References:
- Pest Management Regulatory Agency
- Pest control products (pesticides) acts and regulations
- International best practices on pesticide regulation
- Re-evaluation Note REV2020-01, Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2020-2025
- Pest Management Regulatory Agency 2020 2021 Annual Report
- Registration of Pesticides in Canada under the Joint Review Program
- Pesticide Product Information Database
- Discussion Document DIS2022-01, Further strengthening protection of health and the environment: Targeted review of the Pest Control Products Act


